Review ArticleRadiation Physics
Stanford Experience with Commissioning, Quality Assurance and IMRT/SBRT Treatment of the First Biology-Guided Radiation Therapy
Images
Abstract
Biology-guided radiation therapy (BgRT) is an emerging technology that integrates real-time PET imaging with radiation therapy to improve tumor targeting and treatment outcomes. This systematic review aims to summarize the Stanford experience on the current state of knowledge on machine commissioning, quality assurance, treatment planning, clinical applications, safety, and efficacy of BgRT in cancer treatment. The review underscores advancements in the clinical implementation of intensity-modulated radiation therapy (IMRT) and stereotactic body radiation therapy (SBRT) technologies, facilitated by the introduction of the novel BgRT machine. It also highlights challenges related to improving workflow efficiency and validating tracking accuracy in real-world patient situations. This document serves as a valuable resource for researchers, clinicians, and decision-makers within the realm of radiation oncology, providing insights into the status of the PET-based BgRT machine and guiding the trajectory of future research.
Keywords: BgRT, Commissioning, QA
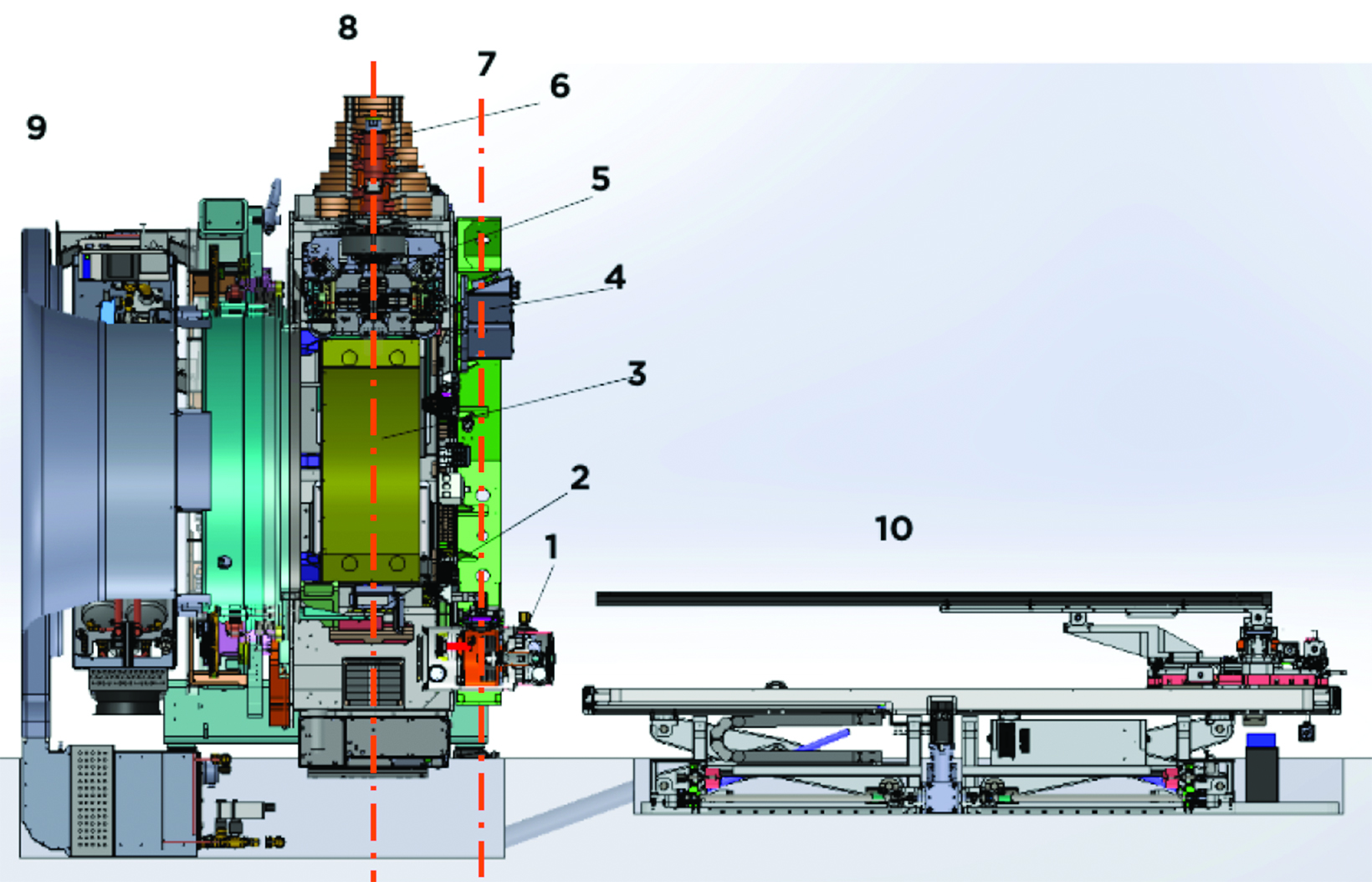
The RefleXion X1 system (RefleXion Medical, Inc.) is a novel PET-guided radiation therapy machine.1-2 The X1 system consists of an 85-cm O-ring gantry linear accelerator (linac) rotating at 60 revolutions per minute (rpm), a fan-beam kilovoltage CT (kVCT) for image guidance of intensity-modulated radiation therapy (IMRT) and stereotactic body radiation therapy (SBRT), and PET for real-time tumor tracking for biology-guided radiation therapy (BgRT).3 Major components of the system are shown in Figure 1. The linac consists of a 6-MV flattening filter-free (FFF) photon beam, a binary multileaf collimator (MLC) with 64 leaves, and 2 pairs of jaws located above and below the MLCs. The width of an MLC leaf is 6.25 mm at the isocenter (85 cm from the source). The maximum opening in the lateral direc- tion formed by all MLC leaves retracted is 40 cm. The jaw pairs open 1 or 2 cm at the isocenter in the longitudinal direction. The nominal beam dose rate is 850 monitor units (MU)/min for the original IMRT/SBRT version of the machine. With the BgRT upgrade, the dose rate is 1000 MU/min. The kVCT scanner is located on a plane 61.4 cm superior to the room laser. The X1 machine consists of 2 symmetrically opposing 90-degree arcs of PET detectors incorporated into the architecture of a ring-gantry at the same plane to the linac 100 cm superior to the room laser.
The treatment delivery with the X1 system is achieved axially with the couch advancing at discrete intervals of 2.1 mm, making 1 or 4 passes through the treated region for IMRT/SBRT and BgRT. Detailed introductions of the X1 system can be found in these publications.4-7 Given the similarities between the X1 and tomotherapy machines, the commissioning processes share much in common. However, the process for the X1 additionally includes small-field measurements and a more extensive imaging system commissioning. The RefleXion X1 system received FDA clearance for conventional kVCT-guided treatment for IMRT/SBRT in March 2020. As of February 2023, the BgRT modality has been FDA-cleared for treating patients with lung and bone tumors, expanding the applications of the system to motion management via PET tracking. Our department was the first to install and commission the RefleXion X1 system for IMRT/SBRT in 2020, utilizing it to treat more than 100 patients since May 2021; we will be the first to upgrade the system to enable BgRT in June 2023. In this report, we present a comprehensive review of the X1 system during its first 2 years of clinical use, including commissioning, quality assurance, treatment planning, machine performance, and initial BgRT clinical trial results.
Commissioning the Machine
The mechanical and dosimetric aspects of the commissioning tests were performed4 according to the AAPM Protocol Task Group 148.8 The imaging system3 and the treatment planning system (TPS) were also assessed.7
Mechanical Commissioning
The mechanical alignments of the radiation source, y-jaw, and MLC were verified using film and ion chambers. A center alignment check in the y-direction was conducted with a 0.3-mm misalignment tolerance. A 2-mm y-jaw opening and ion chamber measurements helped determine source misalignment, with an actual misalignment of 0.049 mm. The x-direction source position was checked against the MLC using a tongue-and-groove test, with transverse profiles measured in a water tank. The out-of-focus value was 0.66%, within the acceptable range. Y-jaw alignment with the beam plane was checked to ensure proper beam intersection and symmetry. Film tests showed y-jaw divergence and twist met tolerance levels of 0.5 mm and 0.5o. Off-axis clinical treatment fields were tested, with center variations within the acceptable range of 0.5 mm. MLC lateral alignment was assessed using a film at the isocenter, and the MLC offset and twist were within tolerances of 1.5 mm and 0.5o. The accuracy of both the couch and laser positioning was verified. A starshot test was conducted to ensure the radiation beams converge accurately at a common isocenter during gantry rotation, yielding a result in which the minimum radius of the tangent circle was 0.7 mm.
Dosimetry Commissioning
Percentage depth dose (PDD) and profile scans were conducted for various field sizes using a diode detector in a compact 3D water tank. The agreement between measurement and TPS calculation was analyzed with 1D gamma analysis. The PDD10 differences were within 1%, with a mean of 0.3% for all fields, and the mean gamma (1%, 1 mm) pass rate beyond Dmax depth was 94.9%. Lateral profiles were measured at various depths, and the measured and TPS modeled transverse profile differences in the field core showed excellent agreement. For all measured fields, the mean profile differences in the field core were -0.3% ± 1.0% and -0.3% ± 1.2% for 2 cm and 1 cm jaw fields, respectively. Longitudinal profiles for fields were measured and compared with the TPS calculation. For all measured fields, the mean and max full-width at half-maximum (FWHM) differences were 0.3 and 0.4 mm for 2 cm jaw fields, and -0.3 and 0.5 mm for 1 cm jaw fields.
Dose-rate fluctuations at different gantry angles were monitored using a TomoDose (Sun Nuclear) diode array, with output constancy at 0.21% and profile constancy within the suggested tolerance. Rotational output constancy was verified with a 0.7% variation using an ion chamber. A synchronicity plan assessed accurate beam transmission through the MLC in clinical step-and-shoot mode with a gantry rotat- ing at 60 RPM and the couch advancing 2.1 mm per step. The film result showed the maximum delivery offset and angular deviations at 0.26 mm and 0.17°, respectively. To assess complex integrated IMRT plan delivery accuracy, the AAPM TG1199 head and neck (HN) and prostate plans were measured using the ArcCHECK (Sun Nuclear) diode array system. The measurement results were compared with TPS calcula- tions via gamma analysis (3%, 2 mm) with the pass rates of 98.2% for the HN plan and 93.4% for the prostate plan.
RefleXion X1’s clinical beams use small beamlets formed by MLC leaves (6.25-mm thick) and narrow y-jaw openings (10 or 20 mm), creating a lack of charged particle equilibrium and making accurate small-field dosimetry crucial. Shi et al6 reported measurements and Monte Carlo (MC) model validation for the first clinical RefleXion unit, covering various small-field sizes. Diode detectors, a W2 scintillator detector, and films were used to acquire PDDs, beam profiles, and relative output factors. Results showed good agreement between diode, film, and MC simulations for output factors, profile penumbra, and FWHM. Averaged beam profile consistency between diode- and film-measured profiles among different depths was within 1.72%. The MC model of the linac, including pre-MLC beam sources and detailed MLC and lower y-jaw structures, was validated using BEAMnrc and GATE simulation codes. The study highlights the importance of ensuring small-field dosimetry accuracy for RefleXion systems, with results demonstrating acceptable consistency and agreement between measurement methods and MC simulations.
Imaging Commissioning
The imaging system, including the kVCT imager and PET imager, were also commissioned and reported. Han et al10 reported on the commissioning of the fan-beam kVCT imaging system for the first clinical BgRT machine, focusing on positioning accuracy, image quality, and dose commissioning. The helical fan-beam kVCT subsystem features a 120-kV x-ray tube and a 16-row gadolinium oxysulfide (GOS) ceramic scintillator detector. A ball-cube phantom was utilized to assess the kVCT subsystem’s positioning accuracy. The Catphan504 phantom (Phantom Laboratory) was imaged to evaluate the kVCT image quality of the BgRT system. The system demonstrated comparable spatial resolution to regular CT simulators through modulation transfer function test results. The evalua- tion demonstrates the kVCT characteristics of the innovative BgRT system, which features an architecture designed to accommodate CT, PET, and a linac. The image quality and HU (Hounsfield unit) constancy are comparable to traditional CT simulators, making the system a valuable tool for online adaptive radiation therapy.
Hu et al3 evaluated the RefleXion X1 machine’s PET subsystem performance using the National Electrical Manufacturers Association (NEMA) NU-2 2018 standard. The X1 machine integrates PET detectors into a ring-gantry linear accelerator, guiding radiation beam delivery. The PET subsystem was assessed based on sensitivity, spatial resolution, count-loss performance, image quality, and daily system checks. Spatial resolution and image contrast were comparable to typical diagnostic imaging systems for larger spheres. Image-quality contrast values were 29.6%, 64.9%, 66.5%, 81.8%, and 81.2%, with background variability of 14.8%, 12.4%, 10.3%, 8.8%, and 8.3% for sphere sizes of 13, 17, 22, 28, and 37 mm, respectively. However, sensitivity and count rate were lower due to the smaller PET detector area in the X1 system. The clinical efficacy of the X1 system in BgRT remains to be validated after it is officially released for clinical use. Overall, the X1 PET subsystem performance is comparable to typical diagnostic PET systems in terms of spatial resolution and image contrast for spheres larger than 13 mm in diameter.
Treatment Planning System Commissioning
The RefleXion X1’s TPS commissioning results, reported by Simiele et al,7 were assessed using multiple phantoms, comparisons with other TPS systems, and representative clinical IMRT and SBRT cases. Dosimetric parameters, output factors, and agreement between TPS and measurements for various clinical plans were analyzed. End-to-end testing with anthropomorphic head and lung phantoms showed total targeting errors of 0.8 mm for isocentric treatments and 1.1 mm for off-axis treatments. Overall, the RefleXion X1 TPS commissioning results were within the tolerances specified by AAPM TG 53, MPPG 5.a, TG 119, and TG 148 for targets greater than a 1.5-cm diameter located less than 15 cm from the treatment isocenter. A subset of the commissioning tests has been identified as baseline data for an ongoing quality assurance (QA) program.
Quality Assurance
A robust QA program is essential for the RefleXion X1, a complex treatment delivery system, to ensure the safety of treatment delivery. Han et al11 reported the annual, monthly, and daily QA measurement results of the first clinical RefleXion X1 machine following the TG-148 guidelines. The daily QA was performed using TomoDose to verify the laser and kVCT alignment, as well as beam output. The daily MV beam output constancy result demonstrated that the machine was stable over a year of operation with a standard deviation (SD) of 1.1%. The mechanical accuracy of the laser, couch shift, kVCT imaging, and MV beam center were all within 1 mm. More comprehensive parameters, including output, beam quality, and profile consistency, were measured monthly using TomoDose and an ion chamber. Monthly TG-51 calibration was conducted, and the machine output was adjusted twice during the first year of operation to maintain the output SD below 0.6%. The monthly mechanical test concluded that the SD from the laser center to the imaging center was 0.64 mm. The kVCT image quality was tested monthly using a Catphan phantom, and the resolution, contrast, uniformity, noise, linearity, HU constancy, and slice thickness of the kVCT remained stable compared with the commissioning image qualities. Dynamic plan deliveries were tested using film, confirming that the deviation from the kVCT imaging center to the MV beam center was within 1mm. The first annual QA included mechanical centering, alignment, and divergence of the source, MLC, and y-jaws. The beam quality and profiles were measured using a 3D water phantom and diodes. All mechani- cal, dosimetry, and imaging tests in the annual QA passed the tolerance suggested by the TG-148. The QA results of the clinical BgRT system provide a valuable reference for future studies on machine stability and operational limits.
Clinical Applications
Treatment Planning Studies
The RefleXion X1 treatment planning retrospective study was conducted by Pham et al5 to evaluate the IMRT/SBRT plan quality and delivery efficiency. A total of 42 patient plans across 6 cancer sites, including conventionally fractionated lung, head and neck, anus, prostate, brain, and lung SBRT, were analyzed. These cases, originally planned with the Eclipse TPS (Varian) and treated with a C-arm linear accelerator, were selected for this retrospective study. For each Eclipse VMAT plan, corresponding plans with different jaw settings were generated on the X1 TPS using the same planning constraints. All clinically relevant metrics, such as planning target volume (PTV) D95%, PTV D2%, conformity index (CI), R50, organs-at-risk (OAR) constraints, and beam-on time were analyzed and compared between 126 volumetric-modulated arc therapy (VMAT) and X1 plans using paired t-tests. All but 3 planning metrics were either equivalent or superior for the X1 10 mm-jaw plans compared with the Eclipse VMAT plans across all planning sites investigated. The Eclipse VMAT and X1 10-mm jaw plans generally achieved superior plan quality and sharper dose fall-off superior/inferior to targets compared with the X1 20-mm jaw plans. However, the X1 20-mm jaw plans were still considered acceptable for treatment. On average, the required beam-on time increased by a factor of 1.6 across all sites for 10-mm jaw plans compared to 20-mm jaw plans and a factor of 5 to 10 compared with VMAT deliveries. The most recent upgrade to 1000 MU/min dose rate can further decrease the beam-on time and the gap between the VMAT and X1 treatment times. The study demonstrated that clinically acceptable IMRT/SBRT treatment plans were generated with the X1 TPS. This indicates that the X1 system can effectively produce high-quality treatment plans for various cancer sites, offering a promising alternative to traditional linac-based treatment planning systems.
IGRT and SBRT Treatment Delivery
The first X1 unit was installed and operated in IMRT/SBRT mode for more than a year. Shi et al12 presented the first-year experience of treating patients in a clinical setting with this system. From May 2021 to May 2022, 78 patients were treated on the X1 system. Clinical and technical data, including treatment sites, number of pretreatment kVCT scans, beam-on time, patient setup time, and imaging time, were collected and analyzed. The most commonly treated site was head and neck (63%), followed by pelvis (23%), abdomen (8%), and thorax (6%). Except for 5 pelvis patients (6%) who received SBRT treatments for bony metastases, all treatments were conventionally fractionated IMRT. The average number of kVCT scans per fraction was 1.2 ± 0.5. The beam-on time averaged 9.2 ± 3.5 minutes, while the patient setup time and imaging time per kVCT were 4.8 ± 2.6 minutes and 4.6 ± 1.5 minutes, respectively. Patient- specific QA results and machine performance were also collected and reported. The patient QA had a passing rate of 97.4 ± 2.8% 3% and 2-mm gamma criteria. The machine uptime was 92% of the total treatment time. The user-satisfaction survey was conducted among 5 radiation oncology physicians, 5 medical physicists, 5 dosimetrists, and 4 radiation therapists to gather feedback on their experience with the X1 system. The kVCT image quality and daily QA process received the highest level of satisfaction, while the treatment workflow for therapists received the lowest level of satisfaction.
Simiele et al13 successfully applied Six Sigma methodology and Failure Modes and Effects Analysis (FMEA) to mitigate errors in IMRT and SBRT treatment planning. The approach consisted of 5 phases: Define-Measure-Analyze-Improve-Control. The multidisciplinary team outlined the workflow process and identified/ranked the failure modes associated with the plan check items using AAPM TG-10014 recommendations. Items with the highest average risk priority numbers (RPN) and severity greater than or equal to 7 were prioritized for automation using the Eclipse Scripting API (ESAPI). The Improve phase consisted of developing ESAPI scripts prior to clinical launch of X1 to improve efficiency and safety. In the Control phase, the FMEA ranking was re-evaluated 1-year post clinical launch. Overall, 100 plan check items were identified where the RPN values ranged from 10.2 to 429.0. Fifty of these items (50%) were suitable for automation within ESAPI. Of the 10 highest-risk items, 8 were suitable for automation. Based on the results of the FMEA, 2 scripts were developed: Planning Assistant used by the planner during preparation for planning, and the Automated Plan Check used by the planner and the plan checker during plan preparation for treatment. After 12 months of clinical use of the X1 and developed scripts, only 3 errors were reported. The average RPN pre-scripts was 138.0 compared with average post-scripts RPN of 47.8 (P < 0.05), signifying a safer process.
BgRT and Clinical Trials
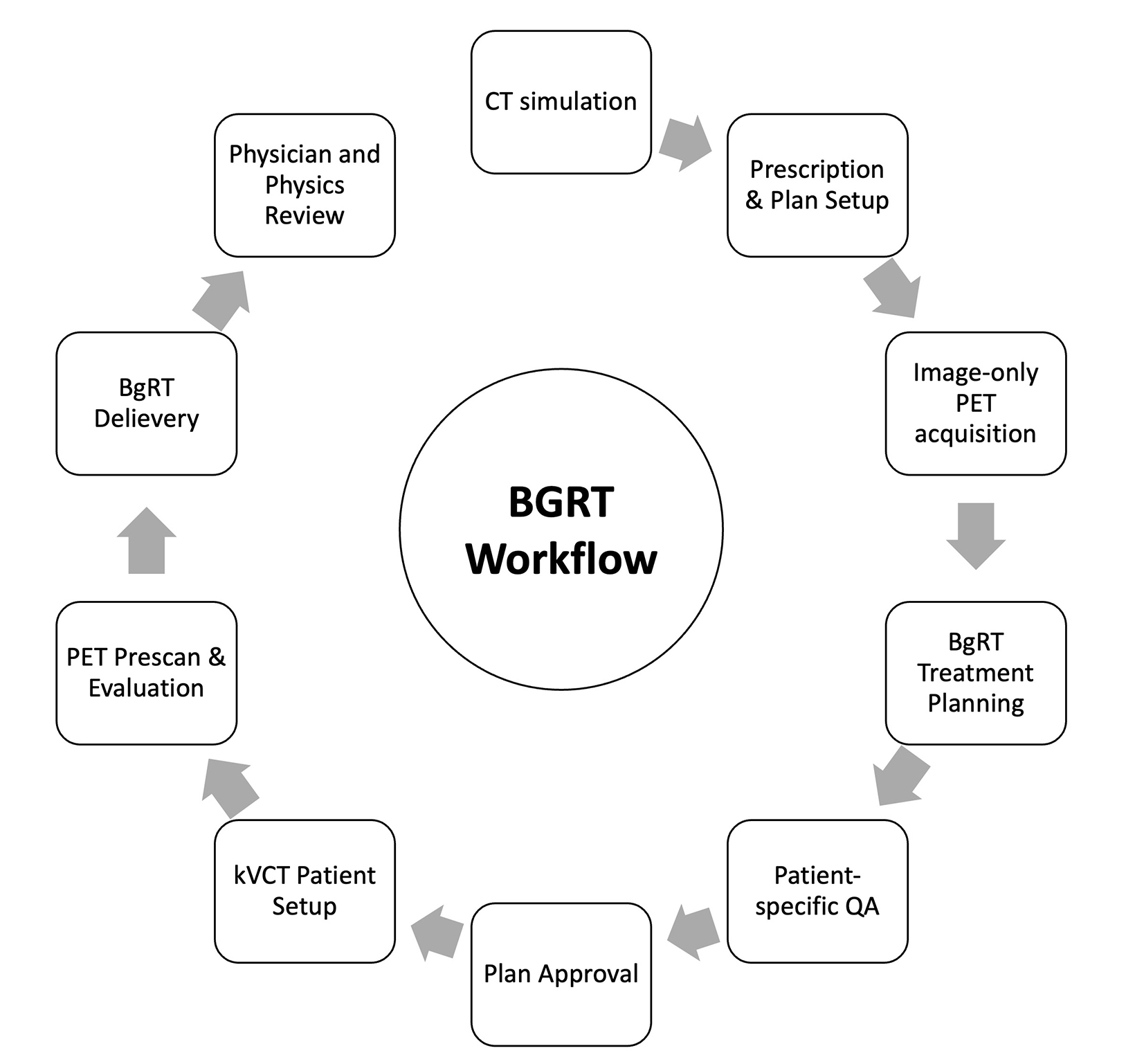
In the first-in-human, multi-institutional clinical trial15 of BgRT, called BIOGUIDE-X, a total of 15 patients were enrolled with the objective of assessing the safety and performance of BgRT. Cohort I aimed to determine whether BgRT plans could be successfully created. Cohort II was designed to assess the deliverability of the BgRT plans on the RefleXion X1 and to further appraise the system’s performance. This was accomplished by obtaining 2 more PET images during the first and last regular SBRT treatment days. The results of this detailed clinical trial will be summarized in future publications. The BgRT workflow steps and time requirements were also assessed in the clinical trial. Figure 2 shows the BgRT process including CT simulation, contouring, imaging-only PET acquisition, BgRT planning, patient-specific QA, plan approval, and delivery. The workflow was assessed by recording time intervals between various steps. The new processes introduced by BgRT were found clinically feasible, but improvements are underway to shorten the time required for each step and increase patient comfort ahead of clinical implementation.
Although the current workflow requires F-18 fluorodeoxyglucose (FDG) administration daily before each BgRT fraction, the recent preclinical evaluation of a PET tracer with a longer decay time, 89Zr-panitumumab (89Zr-Pan)—an antibody PET tracer with a half-life of 78 hours that can be imaged for up to 9 days using PET—was conducted by our group.16 Based on the study analysis translated from mice to humans, BgRT may be feasible for 5 consecutive days after a single 740-MBq injection of 89Zr.
Conclusion
With the recent FDA clearance of BgRT, the department is preparing to treat patients using PET guidance through a new product release, which will improve the current IGRT workflow by increasing the dose rate and decreasing treatment time, improving efficiency of the treatment delivery by providing automated IGRT image matching and enabling re-imaging after large shifts, etc. This 2-year experience with the RefleXion X1 system demonstrates its effectiveness in a clinical setting, offering a promising treatment option for various cancer sites. As the system continues to evolve and incorporate new capabilities such as BgRT, it is expected to further improve patient outcomes and streamline the treatment process.
In conclusion, this review has highlighted the key advancements and findings in the clinical applications of the new FDA-cleared BgRT RefleXion linac. The synthesis of the reviewed studies demonstrates the growing understanding of the complex commissioning, QA, and treatment planning processes. Despite progress, several gaps and limitations in the current literature have been identified, such as optimizing the BgRT workflow and verifying the BgRT tracking accuracy in real patients. To address these issues, future research should focus on PET tracking accuracy, particularly for multitarget treatment. Understanding these aspects will not only advance the widespread use of BgRT, but also broaden its indications for radiation therapy in the treatment of metastatic cancer. Ultimately, continued investigation into PET-based BgRT is crucial for the advancement of radiation oncology as a whole.
References
- Shirvani SM, Huntzinger CJ, Melcher T, et al. Biology-guided radiotherapy: redefining the role of radiotherapy in metastatic cancer. Br J Radiol. 021;94(1117):20200873.
- Oderinde OM, Shirvani SM, Olcott PD, Kuduvalli G, Mazin S, Larkin D. The technical design and concept of a PET/CT linac for biology-guided radiotherapy. Clin Transl Radiat Oncol. 2021;29:106-112.
- Hu Z, Bieniosek M, Ferri V, et al. Image-mode performance characterisation of a positron emission tomography subsystem designed for biology-guided radiotherapy (BgRT). Br J Radiol. 2023;96(1141):20220387.
- Han B, Capaldi D, Kovalchuk N, et al. Beam commissioning of the first clinical biology-guided radiotherapy system. J Appl Clin Med Phys. 2022;23(6):e13607.
- Pham D, Simiele E, Breitkreutz D, et al. IMRT and SBRT treatment planning study for the first clinical biology-guided radiotherapy system. Technol Cancer Res Treat. 2022; 21:15330338221100231.
- Shi M, Chuang CF, Kovalchuk N, et al. Small-field measurement and Monte Carlo model validation of a novel image-guided radiotherapy system. Med Phys. 2021;48(11):7450-7460.
- Simiele E, Capaldi D, Breitkreutz D, et al. Treatment planning system commissioning of the first clinical biology-guided radiotherapy machine. J Appl Clin Med Phys. 2022;23(8):e13638.
- Langen K, Papanikolaou N, Balog J, et al. QA for helical tomotherapy: report of the AAPM Task Group 148. Med Phys. 2010;37(9):4817-4853.
- Mynampati DK, Yaparpalvi R, Hong L, Kuo HC, Mah D. Application of AAPM TG 119 to volumetric arc therapy (VMAT). J Appl Clin Med Phys. 2012;13(5),108-116.
- Han B, Kovalchuk N, Capaldi DP, et al. The kVCT system commissioning of a novel medical linear accelerator designed for biology-guided radiotherapy. Int J Radiat Oncol Biol Phys. 2021;111(3)e532-e533.
- Han B, Shi M, Cui S, et al. One year quality assurance experience of the first RefleXion system. Med Phys. 2022;49(6)E936-E936.
- Shi M, Simiele E, Han B, et al. First-year experience of IMRT/SBRT treatments using a novel biology-guided radiation therapy system. Abstract accepted for ASTRO Annual Meeting; October 1-4, 2023; San Diego, CA.
- Simiele E, Han B, Skinner L, et al. Mitigation of IMRT/SBRT treatment planning errors on the novel RefleXion X1 system using FMEA within Six Sigma framework. Adv Radiat Oncol. 2023;8(5):101186.
- Huq MS, Fraass BA, Dunscombe PB, et al. The report of Task Group 100 of the AAPM: Application of risk analysis methods to radiation therapy quality management. Med Phys. 2016;43(7),4209-4262.
- Surucu M, Vitzthum L, Chang D, et al. TU-430-BReP-F2-4 – Evaluation of measured PET activity metrics from the first-in-human biology-guided radiotherapy clinical trial. AAPM 65th Annual Meeting & Exhibition; July 23-27, 2023; Houston, TX.
- Natarajan A, Khan S, Liang X, et al. Preclinical evaluation of 89Zr-Panitumumab for biology-guided radiation therapy. Int J Radiat Oncol Biol Phys. 2023;S0360-3016(23)00056-1.
Citation
Han B, Kovalchuk N, Gensheimer M, Vitzthum L, Xing L, Surucu M. Stanford Experience with Commissioning, Quality Assurance and IMRT/SBRT Treatment of the First Biology-Guided Radiation Therapy . Appl Rad Oncol. 2023;(2):15-20.
July 4, 2023
